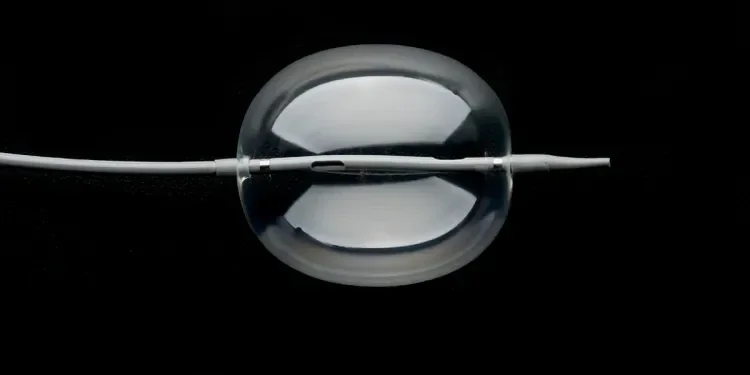
GORE® Molding & Occlusion Balloon
The GORE® Molding & Occlusion Balloon was designed through clinician collaboration to address the challenge of consistently achieving the best possible EVAR stent graft seal and temporary vessel occlusion.
Known as an aortic stent graft innovator, we are committed to making improvements where we can advance the quality and efficiency of care.
This balloon delivers all the performance you need in one balloon that is engineered to positively impact clinical outcomes and provide the best possible experience for the physician and the patient.
The GORE® Molding & Occlusion Balloon provides numerous benefits:
- Optimize seal: Proven radial expansion force across the range of EVAR device sizes (10–37 mm) to seat and seal stent grafts with confidence
- Minimize risk: Engineered with a 10 Fr low profile to reduce access-related complications
- Enhance control: Designed for excellent pushability and trackability with uncompromised inflation/deflation time
Catalogue number: MOB37

INDICATIONS FOR USE IN THE U.S.: The GORE® Molding and Occlusion Balloon Catheter is intended for temporary occlusion of large diameter vessels or to assist the expansion of self-expanding endovascular prostheses (stent grafts).
CONTRAINDICATIONS: The GORE® Molding and Occlusion Balloon Catheter is contraindicated in patients who: are contraindicated to contrast media or anticoagulants; have an arterial entry site that cannot accommodate a 10 Fr introducer sheath; are minors; are pregnant. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
231281777-EN
